Abstract
Background: In the past years, chimeric antigen receptor T (CAR-T) cell therapy targeting CD19 have been shown to be a new and effective treatment option in patients with relapsed/refractory non-Hodgkin lymphoma (NHL) and acute lymphoblastic leukemia (ALL). However, infections with severe and potentially life-threatening complications can be induced by either lymphodepleting chemotherapy and/or the infusion of CAR-T cells, while side effects such as cytokine release syndrome (CRS) might further complicate differential diagnosis.
Methods: Infections and complications were evaluated during inpatient treatment as well as in a follow-up period of the first six months after dosing. Eighty-one dosings of CAR-T cells in seventy-three adult patients with either NHL (88%) or ALL (12%) were analyzed. Bacterial and viral pathogen were detected with blood cultures or examination of potential sources of infection such as catheter tips. Panel and serum testing for viral infection was carried out either as a screening or in case of suspicion, while fungal infections were diagnosed according to the 2008 revised European Organization for Research and Treatment of Cancer (EORTC) Consensus Group criteria to determine proven, probable and possible invasive fungal disease.
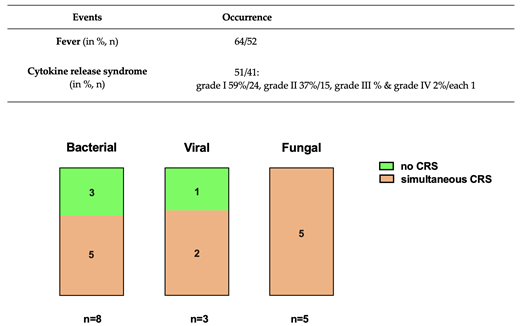
Results: In 52 patients (64%, Table 1) fever was detected following lymphodepletion and CAR-T cell dosing . Microbiological or radiological findings were seen in 20% of cases. Of those, eight were of bacterial (10%), three of viral (4%), and five of fungal (6%) origin. Overall, more lines of therapy as well as more severe CRS were associated with early infection. Cytokine release syndrome was diagnosed in 41 patients (51%, Table 1), with a simultaneous detection in five bacterial (6%), two viral (2%), and five fungal (6%) infections (Figure 1). In the six months follow-up period seven patients with fever (9%) had microbiological or radiological findings, in most cases during the first 90 days after CAR T cell infusion (6 patients, 7%). Only one patient died of infection (pathogen: cytomegalovirus).
Conclusions: Infections are common in CAR-T cell patients; therefore, fast and suitable identification and initiation of treatment are important in the heavily pretreated and immunocompromised patient population. While infectious complications are mostly manageable, the frequency of infectious complications in patients receiving CAR-T cell therapy underlines the value of standardized anti-infective prophylaxis and supportive therapy for reduction of morbidity and mortality.
Schubert: Gilead: Consultancy. Sauer: Pfizer: Consultancy, Speakers Bureau; Abbvie: Consultancy, Speakers Bureau; Matterhorn Biosciences AG: Consultancy, Other: DSMB/SAB Member; Takeda: Consultancy, Other: DSMB/SAB Member. Schmitt: Hexal: Other: Travel grant; TolerogenixX Ltd: Current Employment; Therakos/Mallinckrodt: Research Funding; Jazz Pharmaceuticals: Other: Travel grant. Müller-Tidow: Bioline: Research Funding; Pfizer: Research Funding; Janssen: Consultancy, Research Funding. Dreger: AbbVie: Consultancy, Speakers Bureau; AstraZeneca: Consultancy, Speakers Bureau; Bluebird Bio: Consultancy; BMS: Consultancy; Novartis: Consultancy, Speakers Bureau; Janssen: Consultancy; Gilead Sciences: Consultancy, Speakers Bureau; Riemser: Consultancy, Research Funding, Speakers Bureau; Roche: Consultancy, Speakers Bureau. Schmitt: Bluebird Bio: Other: Travel grants; MSD: Membership on an entity's Board of Directors or advisory committees; Novartis: Other: Travel grants, Research Funding; Hexal: Other: Travel grants, Research Funding; Kite Gilead: Other: Travel grants; Apogenix: Research Funding; TolerogenixX: Current holder of individual stocks in a privately-held company.